How Is an Exothermic Reaction Identified Apex
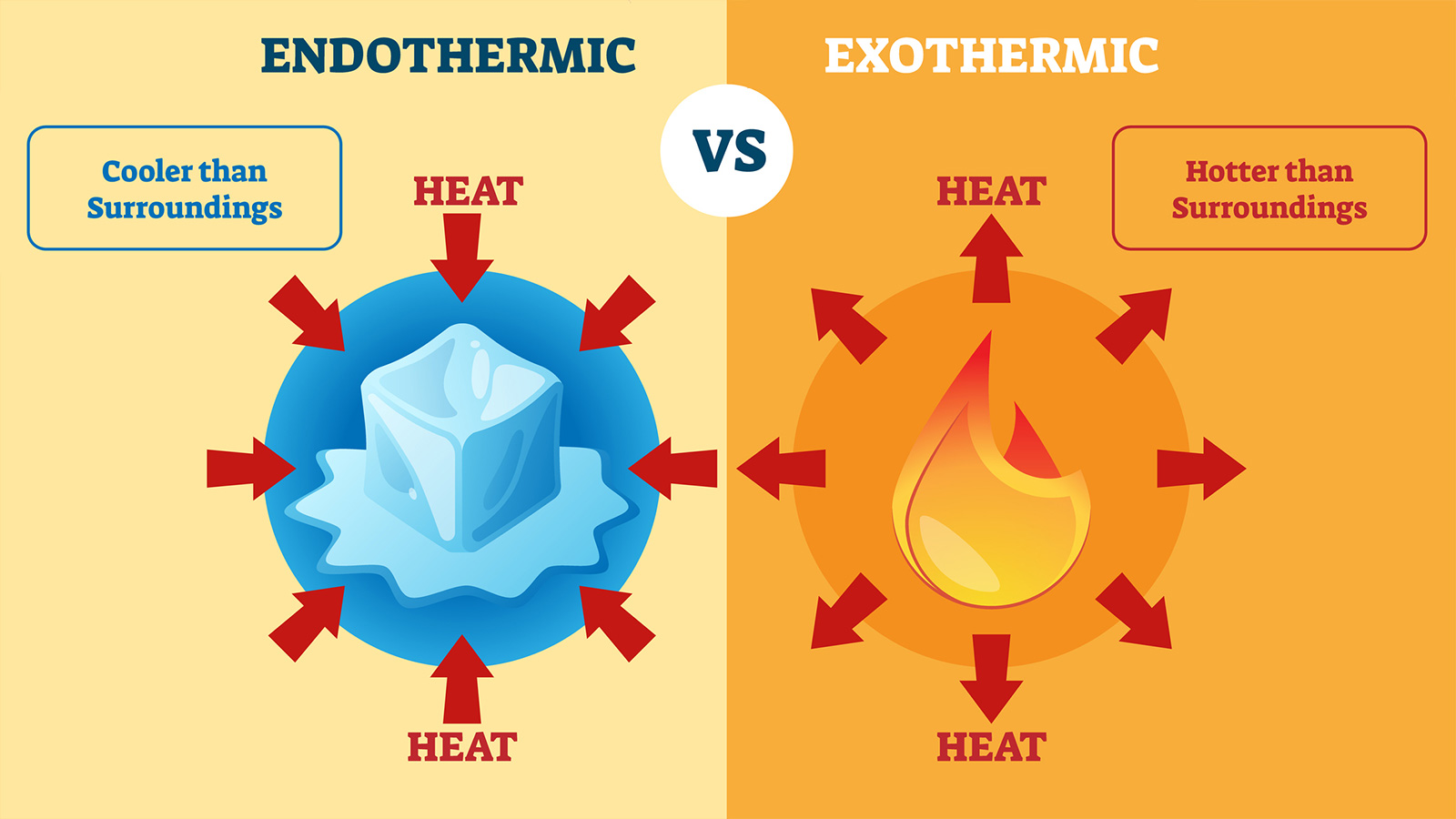
An exothermic reaction is a reaction in which energy is released in the form of light or heat. In the case of exothermic reactions the particular system and the reactants of the system release heat into the surroundings and it cools down.

How Is An Exothermic Reaction Identified Brainly Com
This exothermic reaction can be identified when energy is being released into the.

. A reaction that converts thermal energy to chemical energy heat is taken in Endothermic reaction. It is not a endothermic reaction. One of your salts generated an endothermic reaction with water while the other salt generated an exothermic reaction with water.
Reactants Products Energy. These reactions are energetically favorable and often occur spontaneously but sometimes you need a little extra energy to get them started. Let me first reveal the identity of your salts.
A reaction or system that absorbs heat energy. Exothermic reactions are chemical reactions that release energy as heat or light. In Section II-B of Chapter 3 we defined the heat flow q as negative when heat flows from the system to the surroundings.
IT REQUIRES HEAT AS A REACTANT APEX. Three ways to tell if a reaction is endothermic. Figure1 If the energy produced in an exothermic reaction is released as heat it results in a rise in temperature.
In the case of endothermic reactions the entire system and the reactants gain heat from the surrounding. In other words the activation energy needed to initiate the reaction is less than the energy it releases. The system cools down and you can use a thermometer or your hand to tell that the flask becomes colder.
Thermodynamically though it is absorbing heat energy per. Thats because you were given two different salts. A popular example of an endothermic chemical reaction is photosynthesis.
This answer is. During this process plants absorb energy from the Sun and convert it into. There is an exothermic reaction that occurs when a closed system exists when using a sealed calorimeter.
How is an endothermic reaction identified in an equation. An endothermic reaction is one which absorbs heat or has heat added. So it is usually shown on the reactant left side of the equation.
ΔH represents the change in energy. The word exothermic is derived from exo the Greek word for outside and therme the Greek word for heat. Examples of endothermic changes.
In other words the activation energy needed to initiate the reaction is less than the energy it releases. Keep reading to learn more about exothermic reactions and to identify examples of exothermic reactions in real life. In an exothermic reaction heat is released and it is identified by increase in temperature the material or vessel in which reaction is carried out becomes heated.
It is a exothermic reaction. There is a basic difference between the mechanism and the process of exothermic and endothermic reactions. Exothermic and endothermic reactions.
A reaction that converts chemical energy to thermal energy heat is given out Exothermic reaction. Table 5-1 gives examples of. In reality these systems feel cold to the touch because they are absorbing heat from their surroundings eg.
An exothermic reaction is a chemical reaction that produces heat has a negative ΔH. An exothermic reaction reaching temperatures above 150C can be caused by overload currents or inferior electrical wire connections. Any chemical reaction or change in which energy in the form of heat is released.
Exothermic reactions may occur spontaneously and result in higher randomness or entropy ΔS 0 of the system. 189 The heat treatment above 150C degrades the breakdown properties of PVC cables working in temperatures from room temperature to 90C. An example is the reaction of acetic acid with baking soda.
Salt A is ammonium nitrate and Salt B is calcium chloride. The general equation for an exothermic reaction is. In an exothermic reaction change in enthalpy ΔH will be negative.
They are denoted by a negative heat flow heat is lost to the surroundings and decrease in enthalpy ΔH 0. Thus exothermic reactions have a negative ΔH of reaction. In simple terms the endothermic reactions absorb energy from the surrounding that is in the form of heat.
For example A B heat --- C D. Examples of exothermic changes. An exothermic reaction is a chemical reaction that releases heat and has a negative enthalpy -ΔH and positive entropy ΔS.
Every chemical reaction includes the same components. Combustion neutralisation displacement condensation. Chances are there are examples of exothermic reactions all around you.
How Do They Work. When a chemical reaction occurs energy is transferred to or from the surroundings. On the other hand an exothermic reaction releases energy into the surrounding of the system.
In the lab exothermic reactions produce heat or may even be explosive. Therefore it can be understood that the net amount of energy. Thus in an exothermic reaction energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction.
As a result the products are likely to be warmer than the reactants. Exothermic reactions are chemical reactions that produce heat. There is usually a temperature change.
These are exothermic reactions. On a molecular level chemical reactions happen when the reactant molecules collide with enough energy to break down existing chemical bonds so that new ones can form. 189 This may cause deterioration of insulating properties in PVC due to its chemical decomposition.
Heat will appear as a reactant in the chemical equationCH₃COOH NaHCO₃ heat CH₃COONa.

Enthalpy Changes 3b Flashcards Quizlet

Incisors And Canines Dental Assistant Study Student Life Dental Assistant
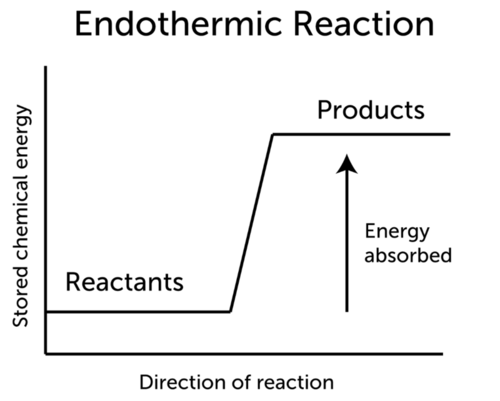
Endothermic Reaction Ck 12 Foundation
Exothermic Reaction Examples Found In Real Life

The Reaction Profiles For The Attack Of O To Ch4 In The Apex A And Download Scientific Diagram
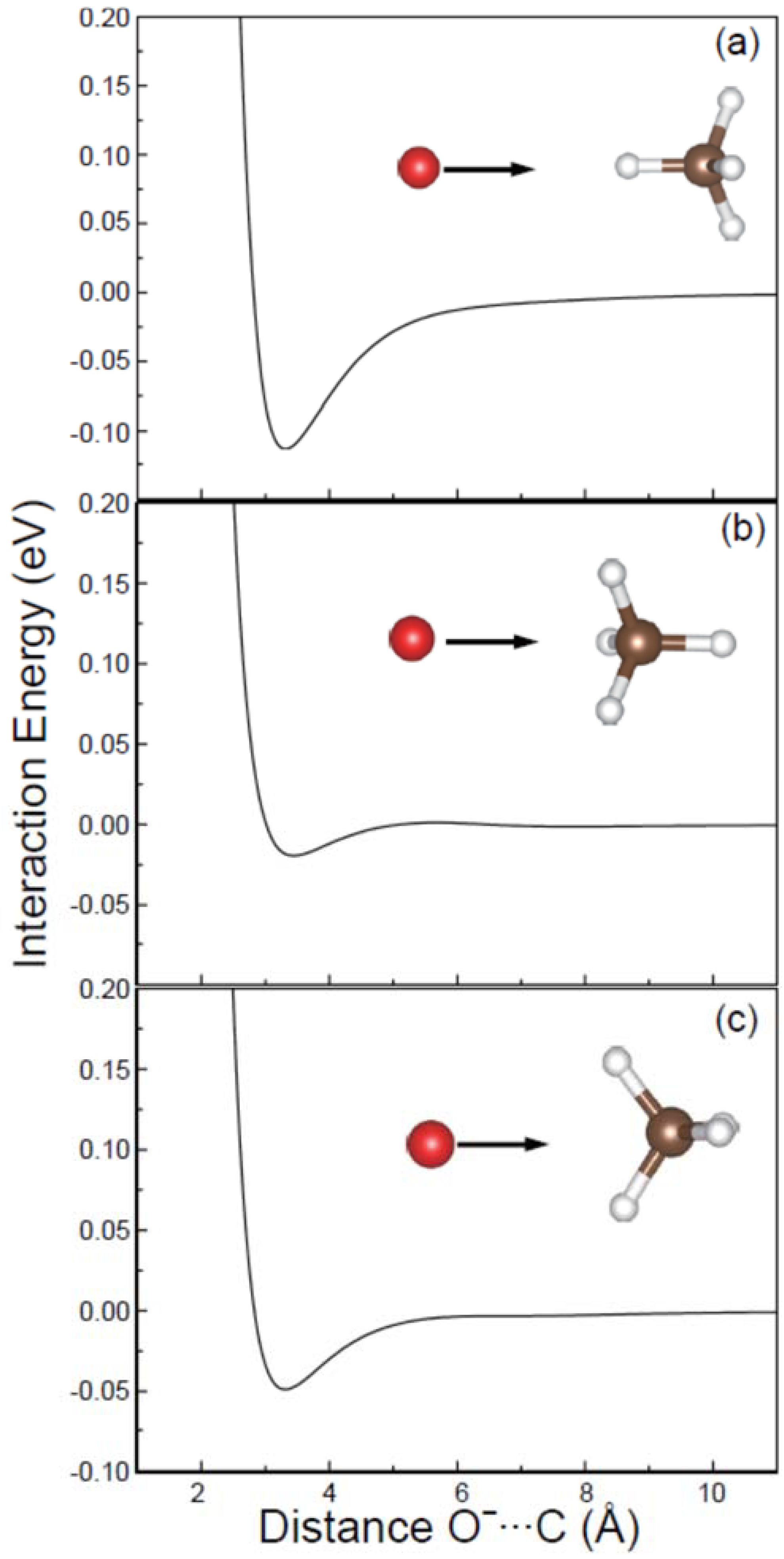
Molecules Free Full Text Ab Initio Molecular Dynamics Simulation Study On The Stereo Reactions Between Atomic Oxygen Anion And Methane Html

Which Of The Following Involves An Endothermic Reaction Brainly Com

How To Memorize The Periodic Table Easiest Way Possible Video 1 Youtube How To Memorize Things Learning Methods Periodic Table

Chem Lab Pdf 32 18 50 Answer These Questions Before Beginning The Lab Be Sure To Turn Them In When You Submit Your Lab Report 1 Suppose You Course Hero

Ncert Solutions For Class 11 Biology Chapter 15 Plant Growth And Development Biology Solutions Class

9 4 3 Lab Heats Of Reaction Pdf Heats Of Reaction Semester 2 Unit 3 Lab 8 Heats Of Reaction Note To Students This Is A Dry Lab You Are Only Course Hero

Activation Energy Endothermic Exothermic Reactions Stock Vector Royalty Free 1488971129

The Reaction Profiles For The Attack Of O To Ch4 In The Apex A And Download Scientific Diagram

A An Endothermic Reaction I Taking Place In A Test Tube What Would You Expect To Feel When You Brainly Com
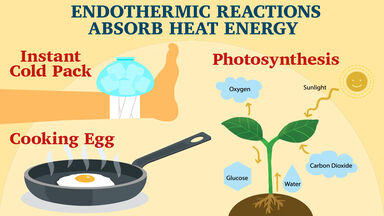
Simple Endothermic Reaction Examples

Endothermic And Exothermic Reactions With Potential Energy Diagrams Youtube

Endothermic And Exothermic Reactions With Potential Energy Diagrams Youtube

Comments
Post a Comment